What Determines How Fast a Substance Will Dissolve
Figure 161 Stirring and heating increase the rate at which a solute dissolves. Molarity M Formula to calculate -moles of solute-.

Which Solids Dissolve In Water Cool Science For Kids
Greater the area greater is the rate of solution.
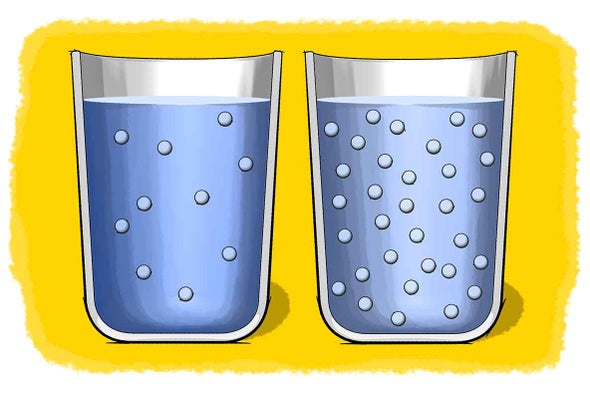
. In the formation of a solution how does the solvent differ from the solute. Factors that determine how fast a substance dissolves are stirring temperature and surface area. The number of moles of solute dissolved in 1 L of solution.
The factors that determine how fast a substance dissolves are stirring temperature and the surface area of the dissolving particles saturated solution contains the maximum amount of solute for a given quantity of solvent at a given temperature and pressure. M1 V1 M2 V2 Formula to calculate -Percent by volume vv volume of solutevolume of solution x 100. Which of the following factors - 5084747 ronalyn1267 ronalyn1267 20102020 Science Elementary School answered How rate of a solution is a measure of how fast a substance dissolves.
When added to water the molecules of polar or ionic compounds break up into ions and form hydrogen bonds. Stirringagitation temperature and the surface area of the dissolving particles determine how fast the substance will dissolve. Water that contains dissolved substances.
How rate of a solution is a measure of how fast a substance dissolves. Factors that determine how fast a substance dissolves are stirring temperature and surface area. Nature of solute C.
The rate of solution is measure of how fast a substance dissolves. More carbon dioxide will remain in solution at the colder temperature found in the refrigerator. 50 rows Stirring agitation temperature and the surface area of the dissolving particles.
Increasing temperature increases the rate of solution. The polarity or ionic property of a compound determines its ability to dissolve in water. A fast solvent is a substance that can dissolve or extract other substances usually without causing chemical changes to itself or the other substances.
This was correct because while observing my data it was always soda that was the fastest with 9 minutes being the shortest dissolving time. The compositions of the solvent and the solute determine whether a substance will dissolve. The factors on which the rate of solution depends are.
Determine whether a substance will dissolve. The amount of solute that dissolves in a given solvent depends upon the temperature and pressure. B the hydroxide ion concentration is less than the hydronium ion concentration.
Size of container B. Water is a polar molecule and transmits a partial positive and negative charge between its atoms enabling it to easily dissolve other ions and polar molecules. Particles area of the solute.
Chemistry 22062019 0330 tbeck225. Stirring agitation tempera-ture and the surface area of the dissolving particles determine how fast the substance will dissolve. Amount of solvent C.
These three factors involve the contact of the solute with the solvent. The dissolved particles in a solution. The rate of solution is a measure of how fast a substance dissolve.
Effect of stirring 1. Effect of temperature D. Formula to calculate -Percent by mass mm mass of solutemass of solution x 100.
Stirring increases the rate of solution. If a solution is considered basic then a the hydroxide ion and hydronium ion concentrations are equal. Hence the correct choice is.
Stirring agitation temperrature and the surface area of the dissolving particles determine how fast the substance will dissolve. A cube of sugar in. 3 Get Other questions on the subject.
Stirring agitation temperrature and the surface area of the dissolving particles determine how fast the substance will dissolve. My hypothesis was that soda was the strongest because it has a lot of carbonation. In conclusion as you can see this project came out well.
Fast solvents evaporate very rapidly under atmospheric conditions. The polarity of a solvent determines the types of substances it can dissolve as well as the liquid substances it can be. The dissolving medium in a solution.
A solvent dissolves the solute. All of the following are factors that determine the rate of solution EXCEPT Stirring B. What determines how fast a substance will dissolve Answers.
If a solution contains less solute than the maximum amount it can dissolve at a given temperature the solution is said to be. The solute becomes dispersed in the solvent. Which of the following is the factors that determine the rate of solution.
The rate of solution is the rate at which a substance dissolves. The solubility of a gas in a liquid increases with decreasing temperature. Solubility is usually expressed in grams of solute per 100 g of solvent.

Solubility Science How Much Is Too Much Scientific American


Comments
Post a Comment